Partner Article
Bradford firm’s rapid Covid-19 test kits approved for professional use
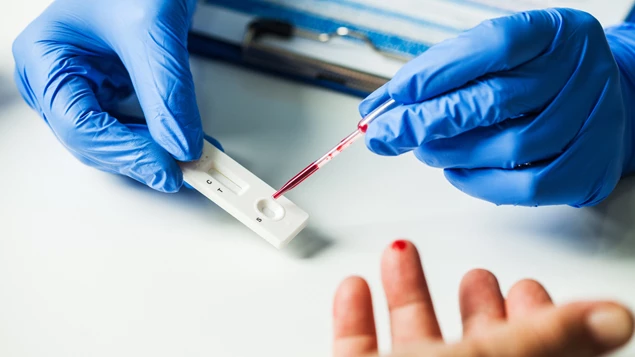
Bradford based manufacturing and distribution company Multibrands International has gained coveted EU and UK regulatory approval for a rapid test kit that shows whether patients and those in social care settings have had Covid-19 Coronavirus - even if they did not develop obvious symptoms at the time.
The point of care test, which is only for use by healthcare professionals, uses a fingertip blood sample to detect the presence of antibodies produced by the body’s immune system that lasts long after the initial infection. A positive or negative result is shown within 10 to 15 minutes.
The test kits have now received coveted ‘CE’ certification for their conformity with all EU medical diagnostic device health protection directives. Product registration approval has also been confirmed by the UK’s Medicines & Healthcare products Regulatory Agency (MHRA).
Rizwana Hussain, Director at Multibrands International comments: “There was some reticence about rapid test kit use during the initial stages of the pandemic as none had received CE certification, reliability wasn’t assured and there was concern about kits aimed at the general public. “We’ve made it very clear that our test is for professional use and believe it to be one of the first of its type to achieve conformity and able to carry the CE logo.”
While not a test for current Covid-19 infection, the rapid test kit achieves an accuracy rate of 94.68% when compared against that achieved by more complex laboratory tests. It can be used to help confirm whether hospitals and care workers, or those in their care, have had the virus. Data collected from test results can then be used to investigate the past spread of the virus or further inform safe working practices.
The test kits are part of a range of COVID-related products available from Multibrands International. Since the start of the Covid-19 pandemic, Multibrands International has invested over £12m into the production of its PPE equipment brands, Panodyne to support the ongoing battle to tackle the virus and stop the spread of infection.
All products in the Panodyne range have been carefully researched and designed in the UK, and the Panodyne brand has since become a trusted UK supplier of high-quality PPE equipment, including certified, medical-grade Type I and Type II R facemasks, hand sanitizers, disposable gloves and other specialist PPE items that are fully tested to the highest European standards.
Rizwana adds: “We have invested over £12m into our Panodyne Range since the outbreak began in China. As we have facilities based in China, we were able to provide early support and also be on the front-foot in predicting a global need. Of course, we never could have predicted a global shut down, but we are proud to be able to able to support the world’s efforts in tackling this deadly virus.”
Headquartered in Bradford, Multibrands International was established in 1998 manufacturing and supplying a range of premium quality, own-brand, everyday FMCG consumer essentials including personal hygiene, and advanced LED household lighting solutions to leading wholesalers and independent buying groups worldwide. The company currently employs over 50 members of staff with additional administration and production facilities in India and China.
This was posted in Bdaily's Members' News section by Cherelle Jones .
Enjoy the read? Get Bdaily delivered.
Sign up to receive our popular Yorkshire & The Humber morning email for free.








 Navigating the messy middle of business growth
Navigating the messy middle of business growth
 We must make it easier to hire young people
We must make it easier to hire young people
 Why community-based care is key to NHS' future
Why community-based care is key to NHS' future
 Culture, confidence and creativity in the North East
Culture, confidence and creativity in the North East
 Putting in the groundwork to boost skills
Putting in the groundwork to boost skills
 £100,000 milestone drives forward STEM work
£100,000 milestone drives forward STEM work
 Restoring confidence for the economic road ahead
Restoring confidence for the economic road ahead
 Ready to scale? Buy-and-build offers opportunity
Ready to scale? Buy-and-build offers opportunity
 When will our regional economy grow?
When will our regional economy grow?
 Creating a thriving North East construction sector
Creating a thriving North East construction sector
 Why investors are still backing the North East
Why investors are still backing the North East
 Time to stop risking Britain’s family businesses
Time to stop risking Britain’s family businesses