US Department of Defense backs Manchester medtech firm with $1.4m funding
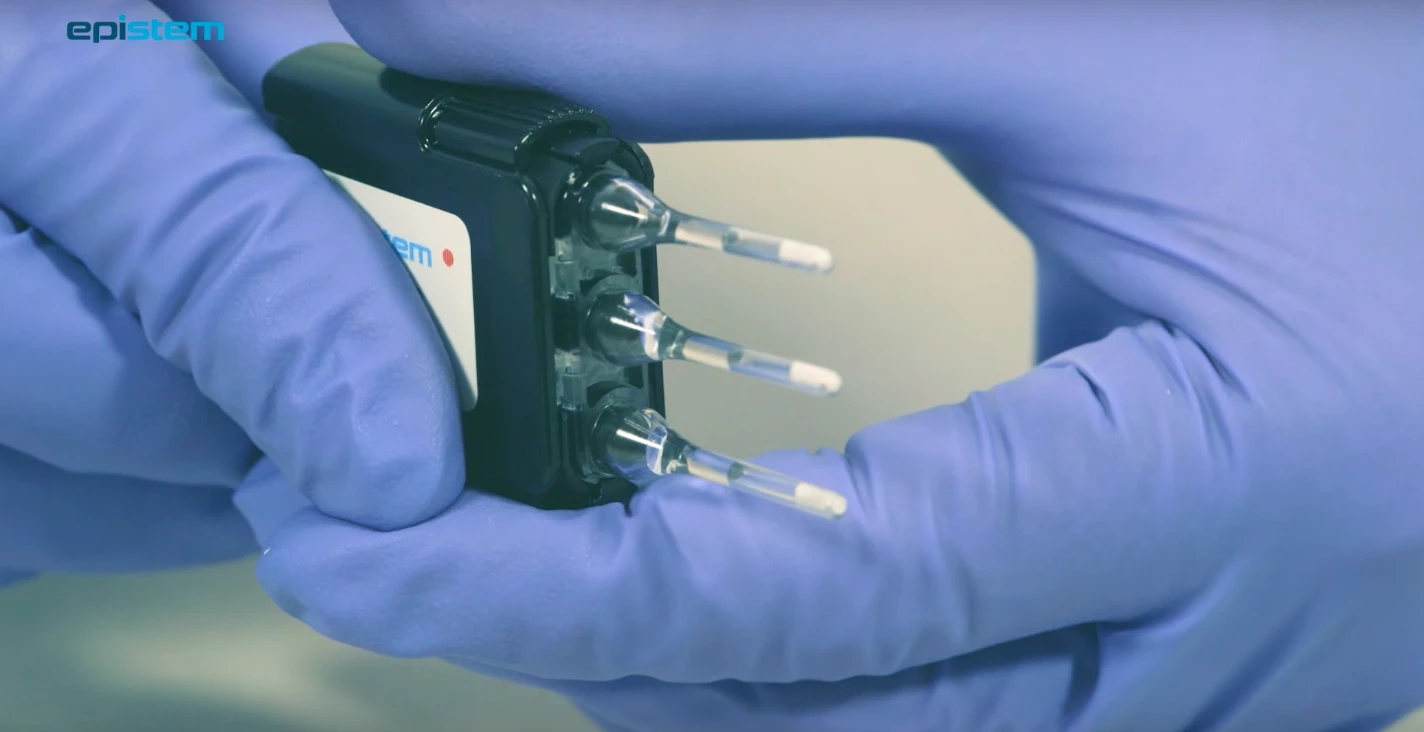
Medical technology business genedrive plc has secured $1.4m (£1.07m) in funding from the US Department of Defense (DoD) for the development of its handheld disease detection device.
The Manchester-headquartered company said the seven-figure boost, for the continuation a DoD programme launched in August 2015, will be used to further develop its small portable Genedrive system.
The firm will enhance Genedrive, which provides molecular diagnostics at the point of need, so that the system can detect a broader range of biohazard targets.
genedrive plc said the ultimate aim of the DoD programme is to develop a cost-effective system allowing multiple units and tests to be deployed for field use.
The new funding brings the total DoD support to $6.5m (£4.95m).
David Budd, CEO of genedrive plc, said: “This next phase of field testing will help to further validate and confirm the characteristics of the Genedrive system: portability, flexibility and accuracy.
“The evaluation will be far reaching, rigorous and, if successful, it will be a clear validation of the Genedrive unit as a robust and easily deployable handheld device for pathogen and disease detection.”
He continued: “During our work with the DoD we have uncovered several developments that have applicability within our proprietary human healthcare initiatives, and, as such, this programme has served to accelerate our own development priorities.”
Looking to promote your product/service to SME businesses in your region? Find out how Bdaily can help →








 The value of using data like a Premier League club
The value of using data like a Premier League club
 Raising the bar to boost North East growth
Raising the bar to boost North East growth
 Navigating the messy middle of business growth
Navigating the messy middle of business growth
 We must make it easier to hire young people
We must make it easier to hire young people
 Why community-based care is key to NHS' future
Why community-based care is key to NHS' future
 Culture, confidence and creativity in the North East
Culture, confidence and creativity in the North East
 Putting in the groundwork to boost skills
Putting in the groundwork to boost skills
 £100,000 milestone drives forward STEM work
£100,000 milestone drives forward STEM work
 Restoring confidence for the economic road ahead
Restoring confidence for the economic road ahead
 Ready to scale? Buy-and-build offers opportunity
Ready to scale? Buy-and-build offers opportunity
 When will our regional economy grow?
When will our regional economy grow?
 Creating a thriving North East construction sector
Creating a thriving North East construction sector