Partner Article
Oval Medical Technologies allergy auto-injector wins praise
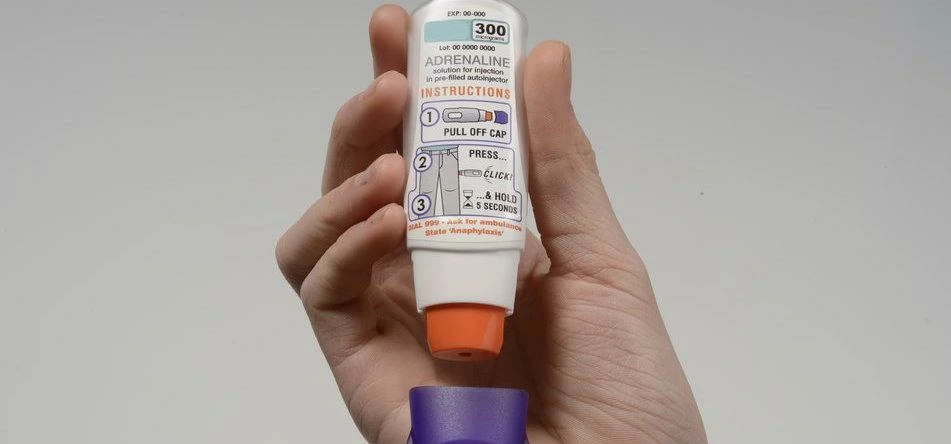
Oval Medical Technologies Limited, a UK developer of medical devices and technologies, has won praise from allergy sufferers and medical professionals for progress in its planned delivery of an easy-to-use adrenaline auto-injector.
An independent study commissioned by the Cambridge company found that all 21 allergy sufferers taking part used the new device successfully without training, demonstrating that simple, clever design could benefit patients.
Seventeen participants said Oval’s compact size was a major advantage, while 19 preferred it to three other auto-injectors available in the market. Portability was a major factor. Many patients preferred Oval as it could fit it in their bag or pocket.
All 10 participants in a companion study of medical professionals preferred Oval’s device to the others – and believed allergy sufferers would carry the device more readily than the other marketed devices.
At 93mm x 29.75mm x 16mm, the slimline Oval device is less than half the size of the current market leader.
Millions in the UK suffer from allergy, with the number of those affected increasing by 5% a year – half of them children. NHS hospitals in England dealt with 20,320 admissions for allergies in the 12 months to February 2014, a 7.7% increase from 18,860 for the previous 12 months. Admissions for allergies were highest in those aged 0 to 4.
Yet many young people won’t carry the potentially life-saving kit used to treat a severe reaction.
Auto-injectors treat early symptoms of an anaphylactic reaction – often caused by a wasp sting or foods such as peanuts. They contain epinephrine (adrenaline) solution to relieve swollen throat and mouth, breathing difficulty and loss of consciousness.
Symptoms such as restricted breathing and falling blood pressure can worsen quickly so those at risk should carry an injector and be able to use it quickly to stave off anaphylaxis.
Health experts are concerned that the size and bulk of injectors – first developed in the 1960s to protect soldiers from biological weapons – can deter young people especially from carrying them and that some struggle to use them properly in an emergency.
In one UK study involving children with severe food allergies, more than half their mothers could not use an injector effectively six weeks after undergoing thorough training. In June 2015 the European Medicines Agency (EMA) directed suppliers to provide more effective educational material for use with their products.
Oval is developing a version of the device that incorporates a longer needle for those who tend to carry fat on their thighs, potentially making an intramuscular injection difficult and reducing effectiveness.
The company is currently undertaking an equity fundraising to complete development of its epinephrine auto-injector and to finalise the manufacturing process for a separate sumatriptan auto injector, and to finance regulatory applications for both.
Barbara Lead, Chief Executive Officer of Oval Medical Technologies, said: “Patients don’t always carry auto-injectors because they’re too big, and they often don’t know how to use them should an emergency occur – parents too.
“Teenagers often won’t carry them at all – but they are more likely to carry a compact device that fits in their pocket. As our report makes clear, size and usability are two crucial factors in persuading patients to carry auto-injectors and ensuring they’re well prepared for an emergency.
“This market research confirms that Oval’s approach to auto-injector design and our progress to date are addressing a vital global healthcare need affecting young people especially. We are now focused on addressing regulatory trials followed by a product launch in about 2019.”
The charity Anaphylaxis Campaign urges all injector users to carry two at all times in case of device failure, making compact size an even more compelling concern.
Lynne Regent, CEO of Anaphylaxis Campaign, said: “In our survey of young people living with severe allergy, only 66% of respondents reported always carrying their epinephrine auto-injectors and we know that one of the reasons is that the devices currently available are too bulky.
“Following the death of an 18-year-old, his parents said he did not carry his allergy injection because it ruined the outline of his skinny jeans. We welcome any new device that would encourage more allergic individuals to carry and to use this life-saving medication.”
Oval Medical Technologies was founded by Matthew Young in 2009 and has achieved a number of milestones in achieving the following aims:
Design an auto-injector that is safe and easy to use in all patient populations, Significantly improve the quality and reliability of auto-injectors and Provide a less contaminating environment for the storage of all drugs.
The company is currently undertaking an equity funding round to support regulatory trials of its epinephrine auto-injector.
For further information please visit www.ovalmedical.com
This was posted in Bdaily's Members' News section by Oval Medical Technologies .
Enjoy the read? Get Bdaily delivered.
Sign up to receive our popular morning National email for free.








 Raising the bar to boost North East growth
Raising the bar to boost North East growth
 Navigating the messy middle of business growth
Navigating the messy middle of business growth
 We must make it easier to hire young people
We must make it easier to hire young people
 Why community-based care is key to NHS' future
Why community-based care is key to NHS' future
 Culture, confidence and creativity in the North East
Culture, confidence and creativity in the North East
 Putting in the groundwork to boost skills
Putting in the groundwork to boost skills
 £100,000 milestone drives forward STEM work
£100,000 milestone drives forward STEM work
 Restoring confidence for the economic road ahead
Restoring confidence for the economic road ahead
 Ready to scale? Buy-and-build offers opportunity
Ready to scale? Buy-and-build offers opportunity
 When will our regional economy grow?
When will our regional economy grow?
 Creating a thriving North East construction sector
Creating a thriving North East construction sector
 Why investors are still backing the North East
Why investors are still backing the North East